Avogadro’s number is considered one of the few fundamental constants in chemistry. By definition, it is the number of Carbon atoms in exactly 12 grams of carbon. A mole of any substance contains an extremely large number of particles and will always be equal to the molar mass of the substance or element.
Calculating Avogadro’s Number. You need values of: – Number of degrees of freedom n – Temperature T – Viscosity “eta” – Radius a – Gas constant R t aN nRT X Y 3 A 2. The Theoretical Equation is. Start by calculating the volume of one mole of solid Al =(26.98 g/mol)/(2.70 g/mL)= 9.99 mL/mol. According to the packing density, only 0.74 og this is atoms (the rest is empty space), so the volume of the atoms alone is (9.99 mL/mol)x0.74 = 7.39.
There are over twenty different methods used to determine the value of Avogadro’s number. In this week’s experiment you will utilize a method that entails counting the number of molecules present in a sample with a known mass.
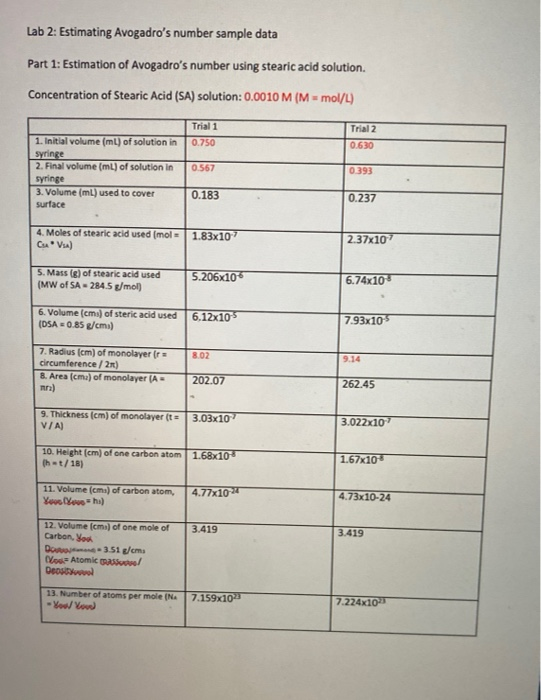
Under appropriately controlled conditions, it is possible to spread a film of fatty substances that are only one molecule thick on a water surface. This single layer is commonly called a monolayer. The thickness of the monolayer can be calculated from the measured volume of the substance required to make the monolayer and the measured area of the surface that is covered. In today’s experiment you will use the fatty acid called Stearic acid to form the monolayer on a bed of water.
You will first calibrate a pipet/dropper in order to accurately measure the volume required to produce the monolayer. Then you will carefully measure the volume of stearic acid needed to form a monolayer. Since you will be given the molecular weight of stearic acid as well as the concentration of the stearic acid solution you will be able to calculate the number of molecules in the monolayer; and from this experimentally calculate Avogadro’s number.
Contrary to the beliefs of generations of chemistry students, Avogadro’s number—the number of particles in a unit known as a mole—was not discovered by Amadeo Avogadro (1776-1856). Avogadro was a lawyer who became interested in mathematics and physics, and in 1820 he became the first professor of physics in Italy. Avogadro is most famous for his hypothesis that equal volumes of different gases at the same temperature and pressure contain the same number of particles.

Avogadro's Law Formula Calculator

The first person to estimate the actual number of particles in a given amount of a substance was Josef Loschmidt, an Austrian high school teacher who later became a professor at the University of Vienna. In 1865 Loschmidt used kinetic molecular theory to estimate the number of particles in one cubic centimeter of gas at standard conditions. This quantity is now known as the Loschmidt constant, and the accepted value of this constant is 2.6867773 x 1025 m-3.
The term “Avogadro’s number” was first used by French physicist Jean Baptiste Perrin. In 1909 Perrin reported an estimate of Avogadro’s number based on his work on Brownian motion—the random movement of microscopic particles suspended in a liquid or gas. In the years since then, a variety of techniques have been used to estimate the magnitude of this fundamental constant.
Accurate determinations of Avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales using the same unit of measurement. This became possible for the first time when American physicist Robert Millikan measured the charge on an electron. The charge on a mole of electrons had been known for some time and is the constant called the Faraday. The best estimate of the value of a Faraday, according to the National Institute of Standards and Technology (NIST), is 96,485.3383 coulombs per mole of electrons. The best estimate of the charge on an electron based on modern experiments is 1.60217653 x 10-19 coulombs per electron. If you divide the charge on a mole of electrons by the charge on a single electron you obtain a value of Avogadro’s number of 6.02214154 x 1023 particles per mole.
Avogadro's Number Practice

Calculating Avogadro's Number Example
Another approach to determining Avogadro’s number starts with careful measurements of the density of an ultrapure sample of a material on the macroscopic scale. The density of this material on the atomic scale is then measured by using x-ray diffraction techniques to determine the number of atoms per unit cell in the crystal and the distance between the equivalent points that define the unit cell (see Physical Review Letters, 1974, 33, 464).
